Marketing authorisation holders, distributors and pharmacies may be facing new obligations to ensure the availability of medicinal products on the Czech market. In fact, on 14 June 2023, the Government approved an amendment to the Medicines Act. The proposal[1] will now be debated in the Chamber of Deputies.
The declared aim of the proposal is to ensure the availability of medicinal products to meet the needs of patients in the Czech Republic and to increase the resilience of the market to shortages of medicinal products.
In order to achieve the above objective, the amendment proposes to introduce three sets of measures:
- obligation on marketing authorisation holders (“MAH”) to ensure additional supplies of a medicinal product for the needs of Czech patients after the notification of interruption or termination of placing the medicinal product on the Czech market;
- labelling a medicinal product with a “limited availability” flag, which will, among others, oblige distributors to supply such a medicinal product to pharmacies within 2 working days; and
- reserve stock system.
It is also proposed to abolish the current protected distribution system (known as “Lex Pawlas”), i.e. (in short) the obligation of the MAH to supply to a distributor who has made a written declaration to the MAH that it requires the medicinal product for patient care in the Czech Republic.
According to its current wording, the proposal should enter into force on the first day of the month following its publication in the Collection of Laws. Taking into account the standard length of the legislative process, it can be estimated that the proposal could enter into force on 1 November 2023 or 1 December 2023. However, it is proposed to postpone the applicability of certain obligations by several months (see below).
Overview of proposed changes
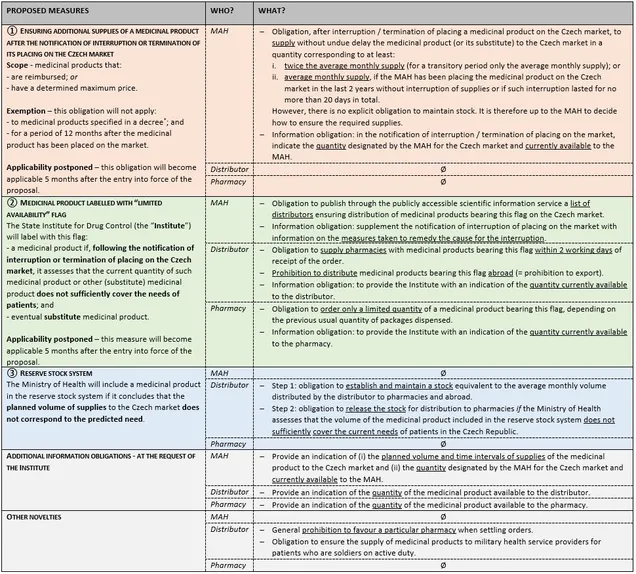
* No draft of this decree is available yet. According to a publicly available outline of this decree, the proposed exemption could apply, for example, to advanced therapy medicinal products, seasonal vaccines, short-expiring medicinal products, radionuclide generators and radionuclide precursors.
How is the parliamentary debate going to change the proposal?
The table above summarises the proposal as approved by the government. The approved wording differs from the original proposal of the Ministry of Health mainly in details and technicalities, despite the large number of comments made during the comment procedure. It remains to be seen whether and how the proposal will be amended during the parliamentary debate.
- [1] – The current version of the proposal can be found here: https://www.psp.cz/sqw/historie.sqw?o=9&t=476.